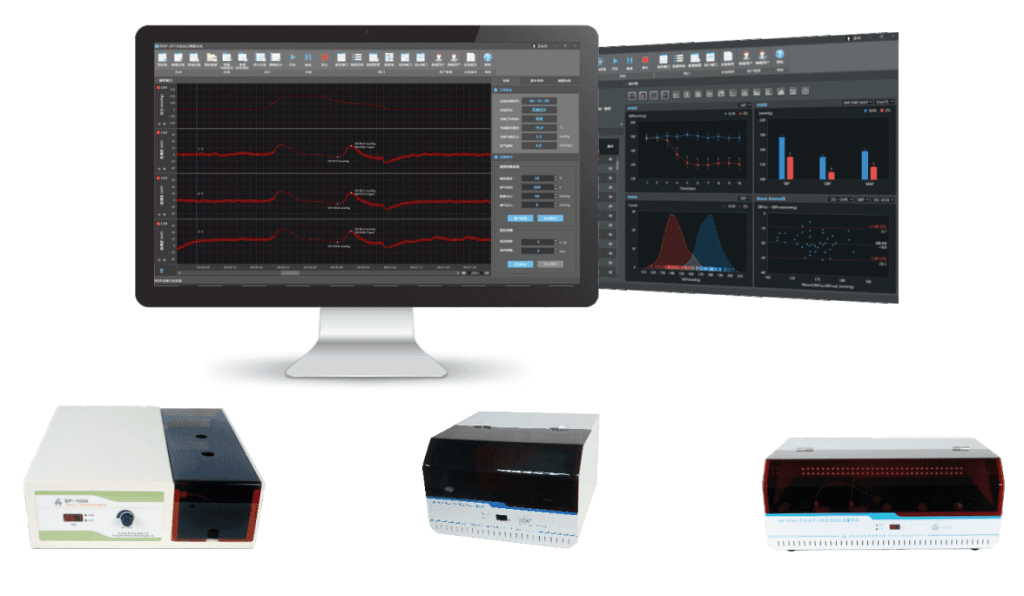
ConductScience introduces the Non-Invasive Blood Pressure Monitor system to measure systemic blood pressure and cardiovascular parameters in mice and rats without any invasive catheterization. Our models can examine single or multiple animals (1-6) at once and can measure the following parameters:
- Systolic pressure
- Diastolic pressure
- Pulse rate
- Mean arterial pressure
- Heart Rate
Specifications
| Features |
TCH-BP-100A |
TCH-BP-300A |
TCH-BP-600A |
| Number of channels* |
1 |
3 |
6 |
| Dimensions |
370mm(L)×360mm(W)×135mm(H) |
400mm(L)×400mm(W)×268mm(H) |
660mm(L)×400mm(W)×268mm(H) |
| Temperature accuracy |
0.1℃ |
0.1℃ |
0.1℃ |
| Suitable animal: |
Rats and mice. |
Rats and mice. |
Rats and mice. |
*The number of channels corresponds to the number of animals that can be monitored simultaneously.
Mouse system
| Appearance |
TCH-BP-100A Quantity |
TCH-BP-300A Quantity |
TCH-BP-600A Quantity |
| PC analysis software |
1 |
3 |
6 |
| MBS43 Basic Accessories |
1 |
3 |
6 |
| SLW20 Mouse Cage |
1 |
3 |
6 |
| SLW27 Mouse Cage |
1 |
3 |
6 |
| SLW40 Mouse Cage |
1 |
3 |
6 |
| MB43 Mouse Tail Pulse Blocker |
1 |
3 |
6 |
| MS43 Mouse Tail Sensor |
1 |
3 |
6 |
Rat system
| Appearance |
TCH-BP-100A Quantity |
TCH-BP-300A Quantity |
TCH-BP-600A Quantity |
| PC analysis software |
1 |
1 |
1 |
| Rat Tail Pulse sensor |
1 |
3 |
6 |
| Tail belt |
1 |
3 |
6 |
| Rat Cage |
1 |
3 |
6 |
ConductScience introduces the Non-Invasive Blood Pressure Monitor system to measure systemic blood pressure and cardiovascular parameters in mice and rats without any invasive catheterization. Our models can examine single or multiple animals (1-6) at once and can measure the following parameters:
- Systolic pressure
- Diastolic pressure
- Pulse rate
- Mean arterial pressure
- Heart Rate
Introduction
TCH-BP-100A/300A/600A fully automatic non-invasive blood pressure measurement system is a computer-controlled non-invasive blood pressure measurement and processing system for rats and mice.
All measurement operations are computer-based. This system is used to observe the arterial blood pressure of animals in chronic experiments. The animals do not require anesthesia, are non-invasive and are easy to use. The system adopts the internationally popular tail-cuff method non-invasive blood pressure measurement principle and is suitable for rats, etc.
The software automatically calculates the mean and standard deviation of the retrieved data, which is shown on a numeric table displayed on the control unit and can be exported to Excel.
Apparatus and Equipment
The Rodent’s Blood Pressure Device consists of a:
- Specimen platform
- Specimen holder
- Tail belt
- Mouse/Rat Cage
- Software
- Power unit
The specimen platform and holder come in different variations for mice and rats. The difference lies in the size of the cages in which the tail cuffs and sensory assembly are larger for rats.
The specimen platform consists of a sensory assembly and tail cuff.
The cuff gradually inflates around the animal's tail when acquiring its blood pressure. Gradual inflation is performed to avoid alarming the animal about ceasing blood flow.
The sensor includes a magnetic top for easy removal while situating the animal. It also makes it more secure once the tail is in place, which drastically reduces tail movement. The tail cuff and sensory assembly are suitable for mice and rats of all sizes without requiring modifications.
The tail cuffs contain a built-in air pump that allows automated inflation and deflation of the cuff at a constant and even rate. Automated deflation of the cuff is performed after each subject reaches their systolic value to prevent the animal's tail from deteriorating from high occlusion pressures. Therefore, real values are achieved and are easily traceable through charting.
The specimen holders for mice have magnetic bases for restraining and positioning the animal quickly. The holders are available in three sizes and can accommodate mice over 60 grams. It can also accommodate neonatal rats.
The specimen tray is used to protect the specimen platform's surface and make cleaning the system easier. Moreover, the animals can be easily transferred to the specimen platform from any loading station using the specimen tray.
The power/utility unit provides power and air to the specimen platform. Each unit can accommodate a single, three and six-channel specimen platform. It includes a universal power supply, making it compatible for international use.
The control unit displays the animal's heart rate, blood pressure, and other numerical data.
The pulse transducer measures the pressure applied to the pressure cuff.
The software controls the specimen platform, determines measurement parameters, initializes measurements, displays waveforms in real-time, and saves results.
Literature Review
A comparative analysis between the retinal neural structure of the hypertensive BPH/2J mouse and the normotensive BPN/3J strain
Herat et al. (2020) compared the retinal neural structure of the hypertensive BPH/2J mouse and its control counterpart, the normotensive BPN/3J strain. The experimenters determined whether the retinal neural phenotype in the BPH/2J strain was because of an increase in blood pressure by investigating the neural retina of both mice strains. Mice (4, 6, 13, 17, or 21-week-old) were used in the study. The Rodent’s Blood Pressure Device was used to measure the animals' blood pressure when they were 6 and 13 weeks. The animals were euthanized after the experiment to retrieve their eye specimen for analysis. Blood pressure results revealed that the systolic, diastolic, and mean arterial blood pressure was greater in hypertensive BPH/2J mice than in the normotensive BPN/3J mice. Analysis of the subjects' retinas revealed severe retinal neural damage in the BPH/2J strain even at 4 weeks. Therefore, the results suggested that high blood pressure may be the cause of the retinal phenotype in the BPH/2J mouse strain.
Investigation of the effect of anakinra in reducing renal inflammation, structural damage, and blood pressure in mice with hypertension
Ling et al. (2017) investigated whether anakinra, a clinically-utilized IL-1 receptor antagonist, can reduce renal inflammation, structural damage, and blood pressure (BP) in mice with hypertension. Male C57BL/6J mice 10-12 weeks were used in the study and were induced with hypertension by uninephrectomy, deoxycorticosterone acetate (2.4 mg/d, s.c.), and replacing their drinking water with saline (1K/DOCA/salt). Anakinra (75 mg/kg/d, i.p.) treatment was commenced 10 days following surgery and was administered for 11 days. The Rodent’s Blood Pressure Device was used to measure the animals' systolic blood pressure. Inflammatory markers, collagen, and immune cell infiltration in the kidneys were measured using immunohistochemistry and flow cytometry. The results indicated elevated systolic blood pressure in the 1K/DOCA/salt-treated mice compared to control mice. Moreover, anakinra decreased the amount of collagen in the kidneys; however, it strangely appeared to worsen the renal and glomerular hypertrophy that accompanied 1K/DOCA/salt-induced hypertension.
Investigation of the effect of ergot alkaloid mycotoxins on the physiological functions and metabolism of mice
Reddy et al. (2020) investigated the effect of ergot alkaloids, ergovaline, and ergotamine, on the physiological functions and metabolism of mice. The blood pressure, including systolic and diastolic pressure, and heart rate of ergovaline and ergotamine-treated mice were measured using the Rodent’s Blood Pressure Device to assess the effect of the mycotoxins. The relative abundances of both ergotamine and its metabolic products in body and brain tissue were determined using metabolite profiling. The animals' motor coordination was also analyzed using an accelerating rotarod. Similar cardiovascular effects were seen between ergometrine and ergovaline in which there were elevations in blood pressure and reduced heart rate. The results also revealed that despite no changes in blood pressure, bradycardia was maintained at low ergovaline levels. Ergotamine was found in the kidney, liver, and brainstem but not in other parts of the brain, indicating that the toxin has effects that are specific to each of these organs. The rotarod test revealed no significant impairment in motor coordination 50 min post-treatment.
Strengths
The Rodent’s Blood Pressure Device allows the non-invasive measurement of rodents' blood pressure and heart rate. It provides accurate heart rate and blood pressure monitoring without any pain, injuries, embolism, or infection that are common with the long-term use of invasive systems, such as the A-line catheter. The device can be used on a single animal or up to eight animals. The device comes in separate versions for mice and rats with holders of various sizes, ensuring that each animal is properly secured to the device according to its size and weight. The holders are specifically designed to reduce stress in the animals. The tail cuff automatically inflates and deflates, making it easier for the experimenter to retrieve the animal's blood pressure while avoiding injury after the systolic pressure is reached. The software included with the device determines measurement parameters and performs several other tasks. The device can obtain fifteen measurements per mouse or rat in less than fifteen minutes
Summary
- The Rodent’s Blood Pressure Device is an apparatus used to measure the blood pressure and cardiovascular parameters of rodents non-invasively.
- It consists of a control unit, pressure cuff, pulse transducer, restrainers, heater, and software.
- The control unit displays the animal's heart rate, blood pressure, and other numerical data.
- The device measures rodents' systolic, diastolic, mean arterial pressure, and pulse rate.
- The software controls the specimen platform, determines measurement parameters, initializes measurements, displays waveforms in real-time, and saves results.
- The device has a dual platform operation, allowing one user to work with up to eight animals. It also allows the blood pressure of different species to be measured with one device using two platforms (one for each species).
- The Rodent’s Blood Pressure Device can be used to measure rodents' blood pressure for several experiments, including studying hypertension in different rodent strains and the effect of drugs and toxins on blood pressure.
References
- Herat, L. Y., Magno, A. L., Kiuchi, M. G., Jackson, K. L., Carnagarin, R., Head, G. A., Schlaich, M. P., & Matthews, V. B. (2020). The Schlager mouse as a model of altered retinal phenotype. Neural regeneration research, 15(3), 512–518. https://doi.org/10.4103/1673-5374.266069
- Ling, Y. H., Krishnan, S. M., Chan, C. T., Diep, H., Ferens, D., Chin-Dusting, J., Kemp-Harper, B. K., Samuel, C. S., Hewitson, T. D., Latz, E., Mansell, A., Sobey, C. G., & Drummond, G. R. (2017). Anakinra reduces blood pressure and renal fibrosis in one kidney/DOCA/salt-induced hypertension. Pharmacological research, 116, 77–86. https://doi.org/10.1016/j.phrs.2016.12.015
- Reddy, P., Hemsworth, J., Guthridge, K. M., Vinh, A., Vassiliadis, S., Ezernieks, V., Spangenberg, G. C., & Rochfort, S. J. (2020). Ergot alkaloid mycotoxins: physiological effects, metabolism and distribution of the residual toxin in mice. Scientific reports, 10(1), 9714. https://doi.org/10.1038/s41598-020-66358-2